Everyone uses smartphones, laptops and wearable gadgets. The big problem for all these devices is the uptime. The performance of the devices is growing and so does the power consumption. Built-in batteries just cannot catch up. And what about other smart devices? Some smart home devices can be plugged into the power grid. These are, for example, network routers, surveillance cameras, metering and control devices (light, heating), etc. But many IoT devices aren’t designed to access the power network. They must use autonomous power sources. Let's see what the options may be.
Power storage, types of batteries, and principles of their operation
If the Internet of Things devices or sensors are stationary and their location is not subject to change, they can be connected to a powerline. Here, AC/DC converters with voltage transducers come into play. The main requirements for such power supplies are compact size, high efficiency, and stability of the output parameters: current and voltage. Converters should be designed for very low power consumption, typically 1–20 watts or less. The market now offers a huge variety of out-of-the-box solutions with parameters that are just getting better each year.
When IoT system cannot be connected to the power network, for example, when remote sensors are involved, something else must be used. The simplest option is to use galvanic cells, or, in other words, batteries and accumulators, as in wearable devices.
But there is one important difference for IoT devices – mostly they consume much less power. Microchips in smartphones, smartwatches and similar things are made with "thin" technologies (7-14 nm), so they work at high frequencies, have high performance but need a lot of energy to run. Modern IoT devices use Systems on Chip (SoC), which are manufactured using "thick" 120-140 nm tech processes. But in this case, instead of pure silicon, a substrate of layered Silicon on Insulator (FD-SoI) structure is used. Such chips are free of parasitic capacities, and hence have smaller leakage currents as compared to the conventional processors. These microchips do not consume or consume very little when not in active use. Naturally, one might expect a device built on such a SoC to run longer on battery. Of course, there will be minor issues with industrial design and approval, as very low consumption requires high-precision measuring devices (e.g. nano- or pico-ammeters) to test. But the main problem is related to the batteries from which the devices will be powered.
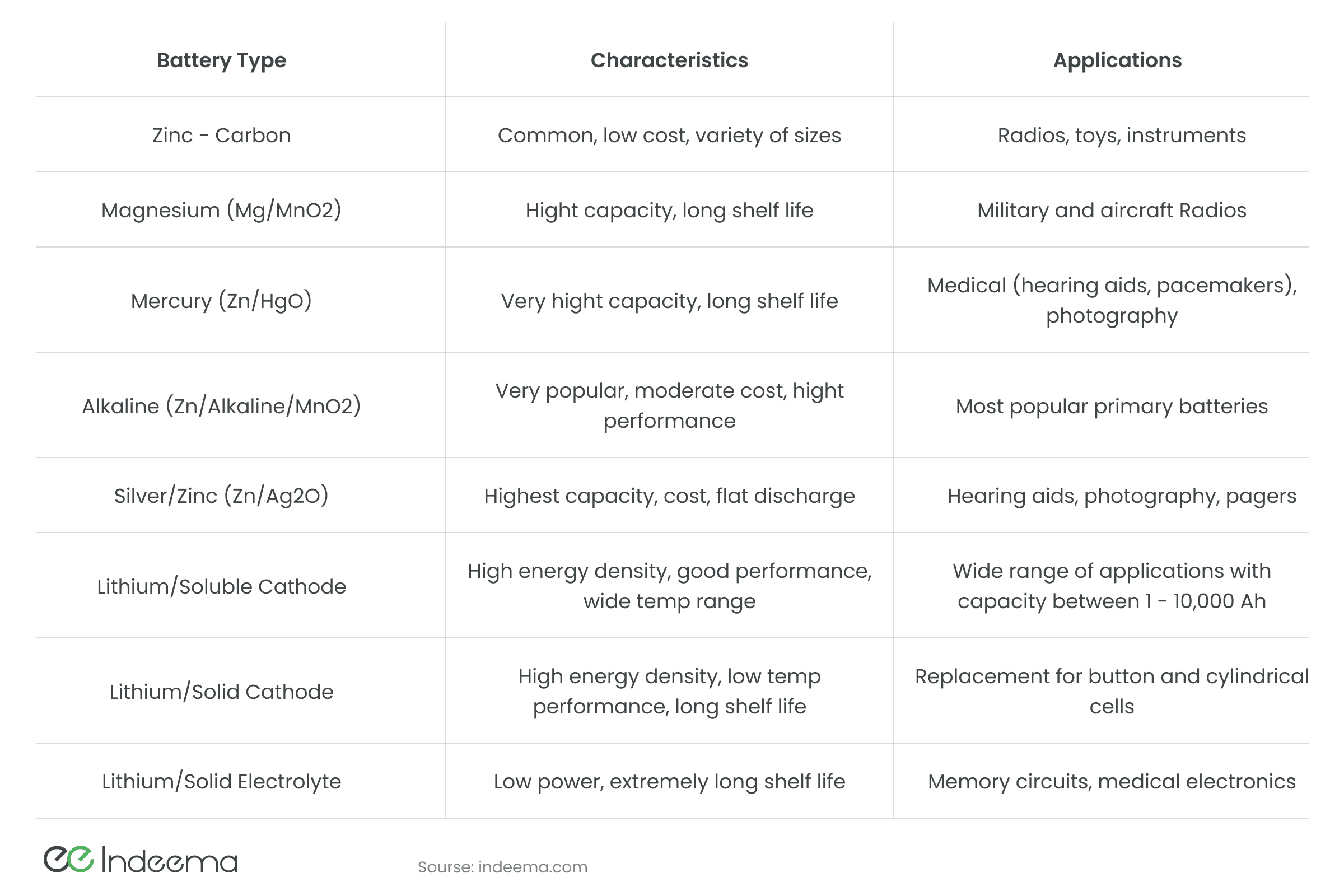
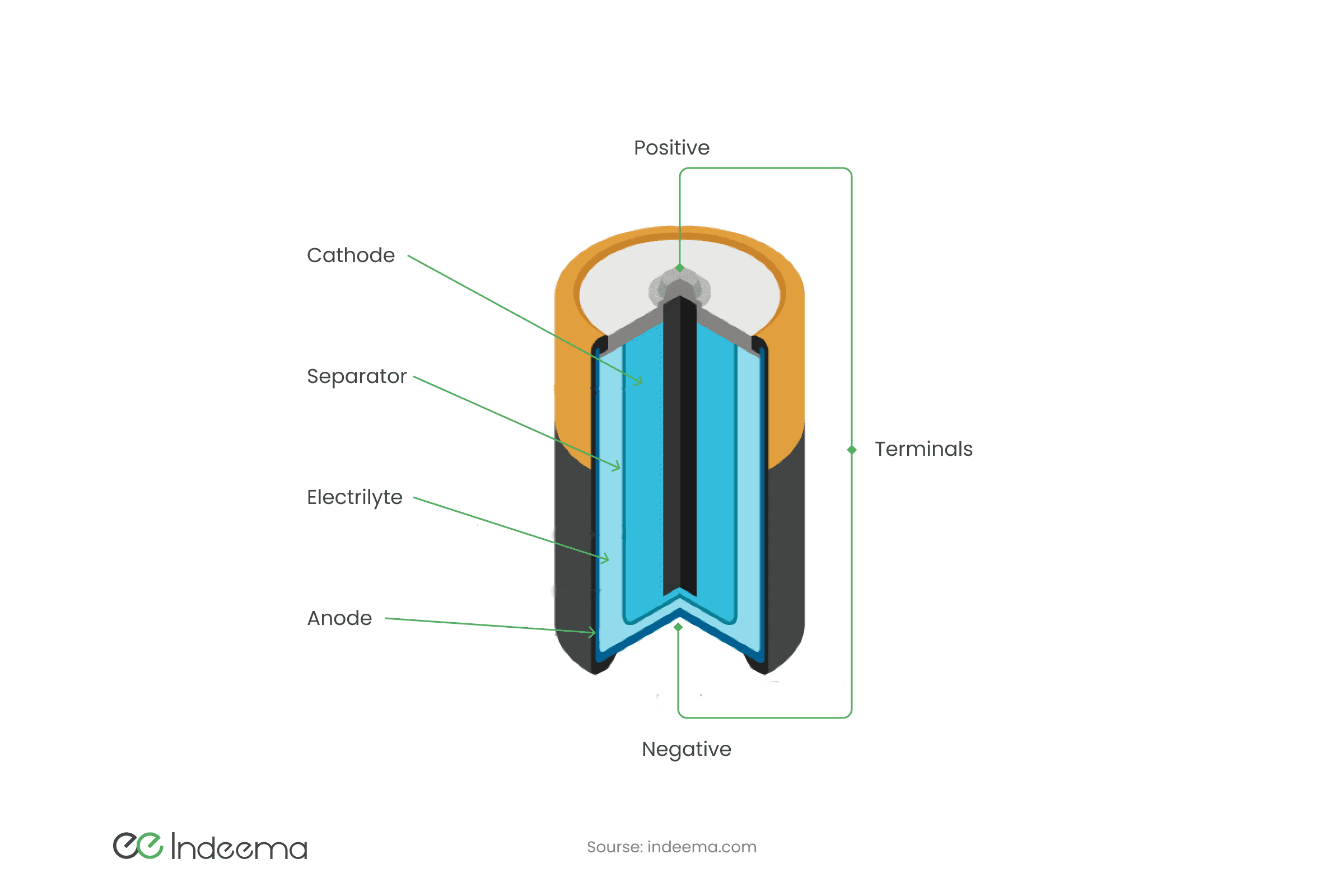
At the heart of a conventional battery is a chemical reduction reaction. There are recharged batteries and those in which the chemical reaction is irreversible. Normally, batteries consist of cathode and anode electrodes (of different materials always) and electrolyte - a substance in which there are free charges moving between the electrodes. If we connect the load to the electrodes, a chemical reaction occurs between the electrodes and the electrolyte, producing an electromotive force. Depending on the materials of the cathode, anode, and electrolyte, there are different batteries and accumulators.
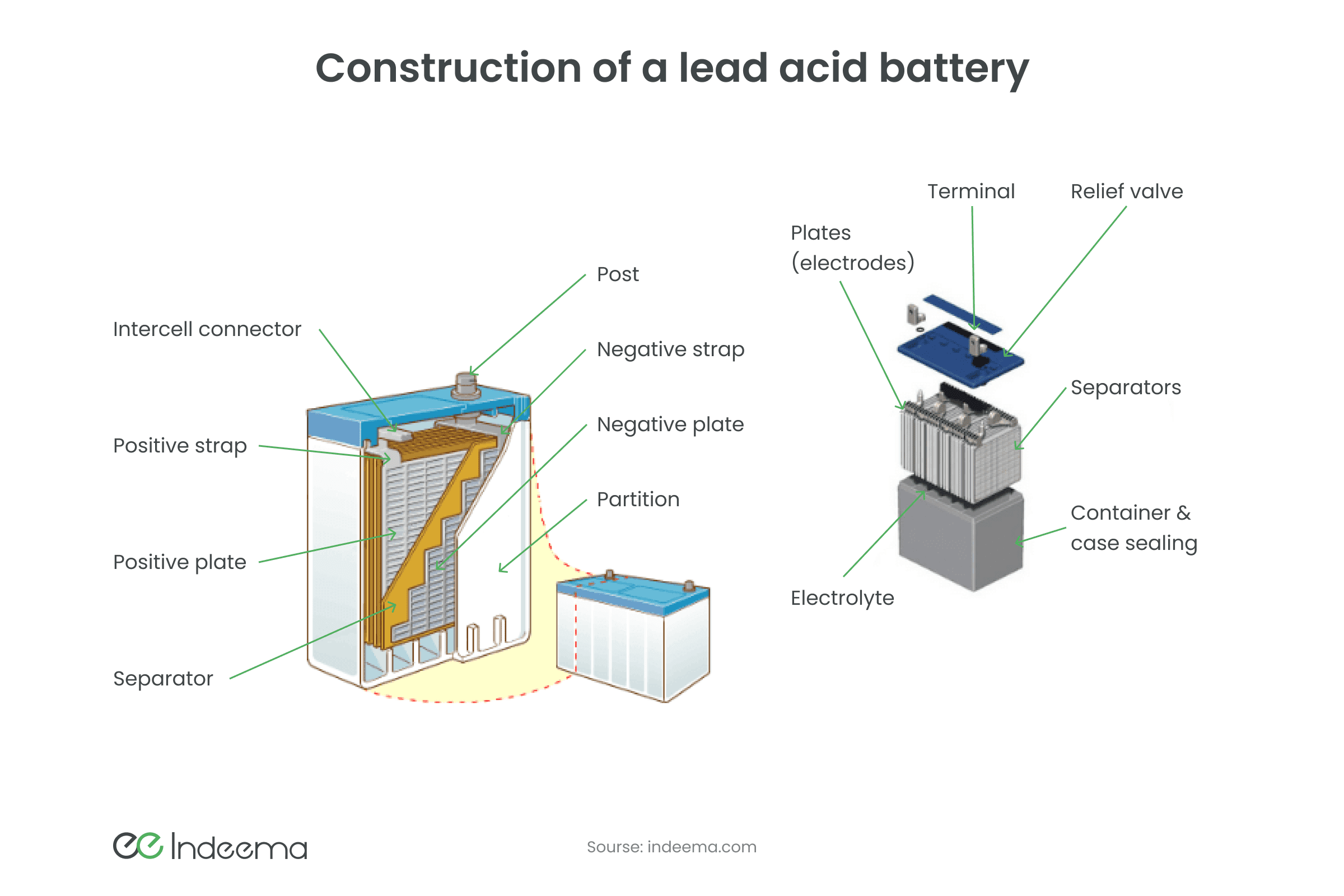
Lead-acid batteries are still the most popular. As the name implies, the electrolyte in it is an acid, or to be accurate, a solution of sulfuric acid; and the electrodes are made of lead (anode) and lead oxide (cathode). These batteries can generate high peak power because of low internal resistance. However, weight and hazardous chemicals inside restrict their use in portable devices. To ensure safety, lead-acid batteries are sealed and a gelling agent is added to the electrolyte.
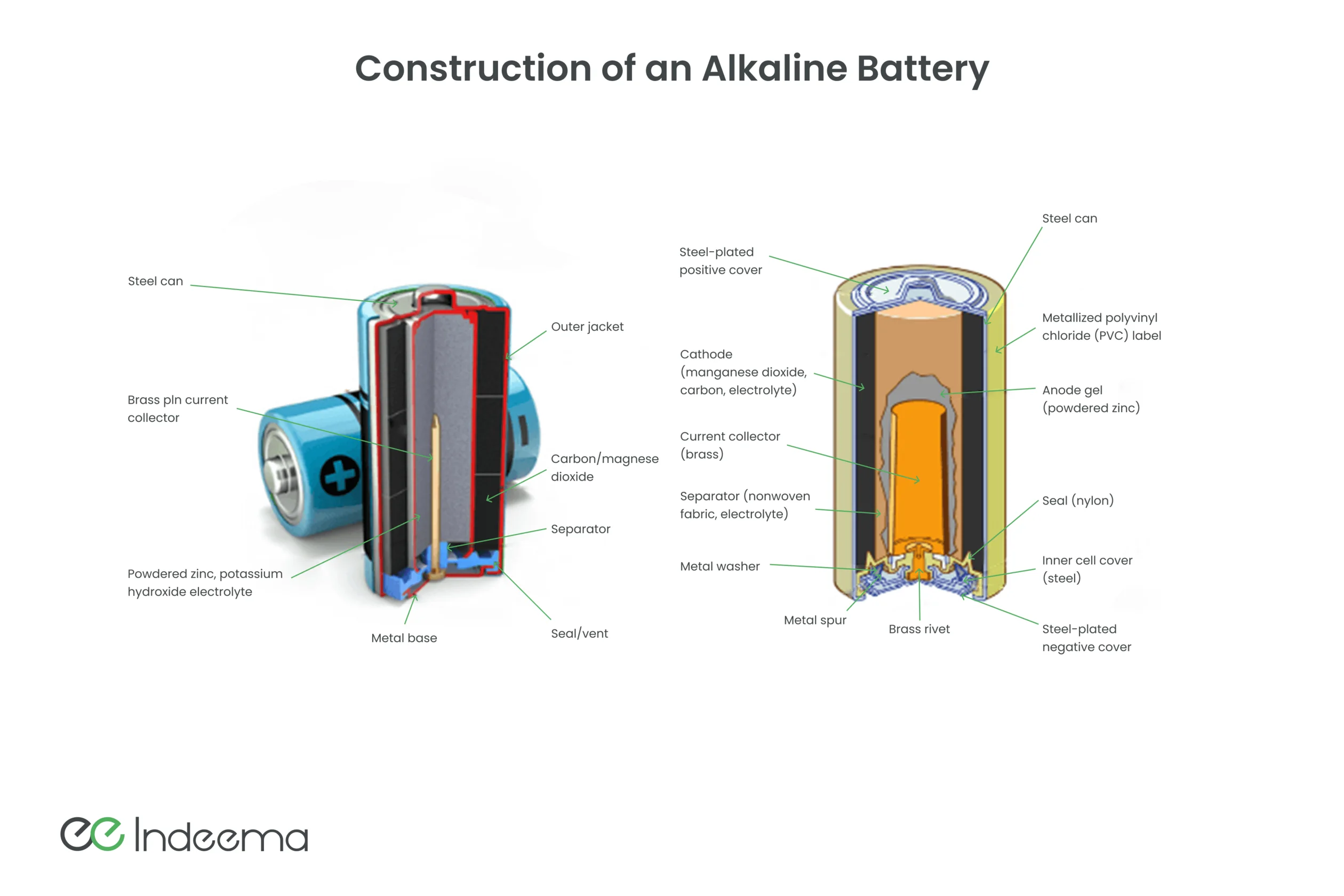
Alkaline batteries and accumulators are widely used in everyday life: they are found in TV or air conditioning remotes, in wireless manipulators, quartz watches, etc. The electrolyte in such batteries is alkali (NaOH or KOH), and the electrodes are made based on nickel and iron. Cadmium was once used, too, only to be banned later for environmental safety. The problem with alkaline batteries has always been their short life, even though their service life is longer than that of salt batteries. Alkaline batteries have a high internal resistance, which leads to high self-discharge and the inability to obtain much power from them. The problems can be partially solved with the help of complex technology for intercalating hydrogen into iron. Alkaline galvanic cells also include silver-zinc batteries. Large capacity to weight ratio and low self-discharge are their main features that allow these batteries to provide energy for low-power electronics for a long time.
Lithium-ion batteries are now the main energy source for electronics. Over the past 6 years, the production of lithium batteries has increased more than 10 times, which led to an increase in the cost of lithium, and hence an increase in the self-cost of batteries. Such batteries are wrapped in aluminium and copper foil, with a porous substance inside that is saturated with lithium electrolyte. This electrolyte consists of a base (graphite, oxide, or salt of metal) into which lithium ions are intercalated. Such batteries have a high energy density, can often be recharged, but age over time (undergoes a non-reducing chemical reaction).
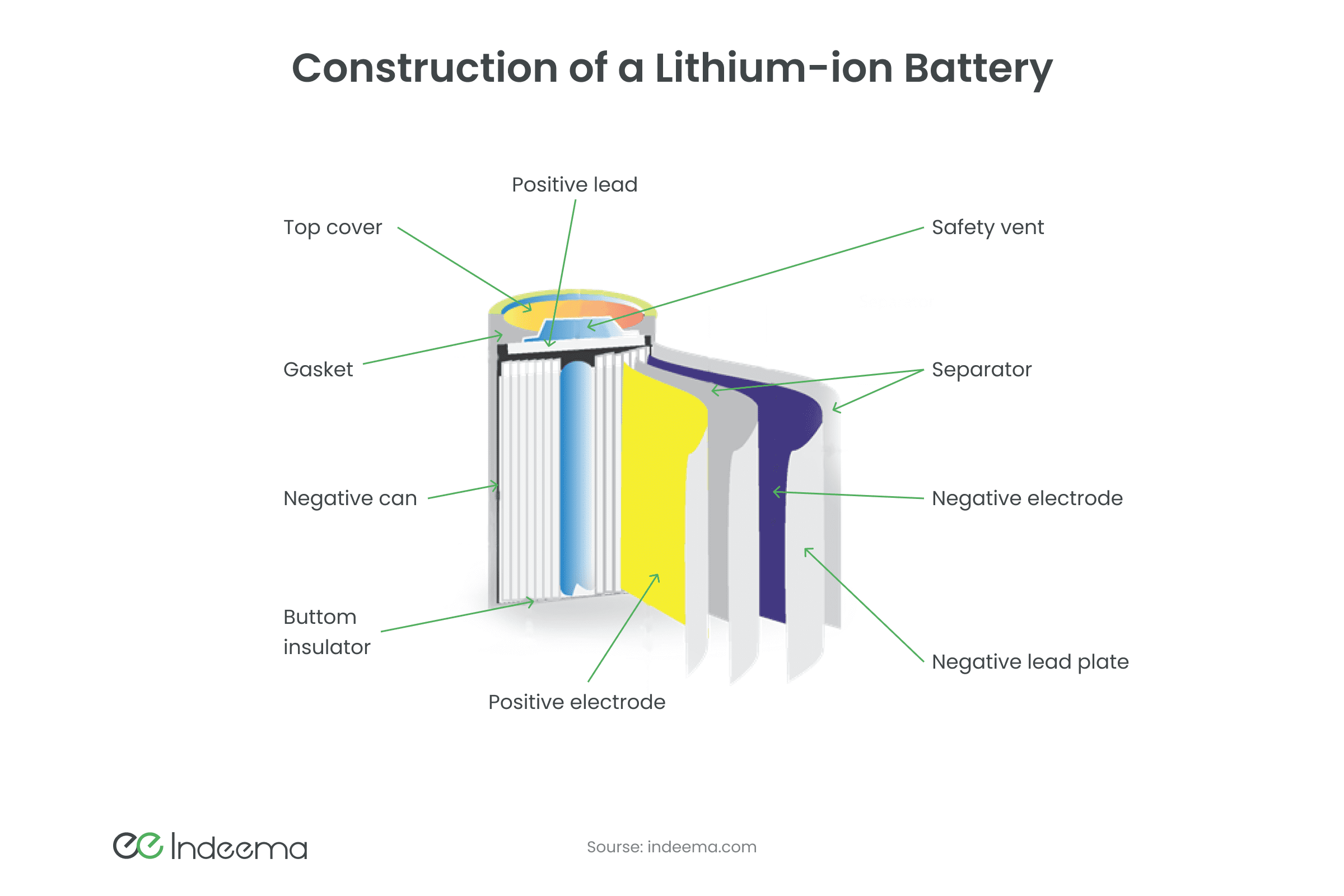
A major disadvantage of chemical galvanic cells for IoT is self-discharge. It occurs due to a chemical reduction reaction and does not depend on external consumption.
Alternative power supplies
In addition to network power converters and galvanic sources, there are other options that can drive the Internet of Things. Alternative energy sources are mostly low power and not applicable for high-capacity production, but often can provide what’s needed for an IoT product.
Solar panels. The most widely used alternative source is solar panels, which use the photovoltaic effect in semiconductors. A quantum of light that hits the surface of a semiconductor can provide energy to an electron that "jumps" to another energy level and can move freely. To do this, the energy of the light quantum must be greater than some value, which is a characteristic of the semiconductor material. It is clear that the effectiveness of such sources will depend on the incident light characteristics, most importantly, on the wavelength.
Thermoelectric effect. This type of source can be based, for example, on the Seebeck effect. When there’s a temperature difference on the opposite sides of a heterogenous conductor, electrons and ions migrate from the hot side to the cold side and develop an electromotive force. Connecting a few such conductors in series and keeping ends at different temperatures, one can accumulate enough EMF to power on an electronic device.
Currents in antennas. We have already talked about radio tags (RFID), where microchips are powered by the electromotive force that is developed in the antenna under the action of the electromagnetic field of the reader. Further development of this idea is to create power sources based on a wideband antenna, which can generate currents from different external fields. However, such sources are effective only in places with significant electromagnetic radiation and are completely unsuitable for normal outdoor use.
Atomic batteries. At the core of those are radioactive isotopes. They are rarely used in everyday life but have found their application, for example, in space programs to power autonomous satellites. The design of isotope batteries with nanodiamonds in the structure is promising for the use in IoT devices. Nuclear power supplies are very durable and can consume power for years, decades, and even centuries. Also, partially recycled nuclear waste is used for their production, which is not that damaging from the ecological viewpoint. The problem with nuclear batteries is their unceasing operation (you need to constantly draw power), and the small current that can be obtained from such a source. Therefore, the device that will use the atomic battery must have an accumulator, such as a chemical battery, that will store power and release it to power the device when needed.
Application in IoT
Depending on the device that belongs to the Internet of Things, it may be appropriate to use different power sources and combinations thereof. For example, AC/DC converters connected to a common power network are typically exploited for energy management devices. Video surveillance systems, robotic cameras, security and access control systems, in addition to the main power supply must also have emergency power. Lead-acid batteries are most often used for this because they can stay constantly charged for the longest time. Other galvanic cells can quickly lose power at a constant 100% charge.
Sensors that collect environmental information consume little power. And since reliable information, for example, about the room temperature is not required to be updated at high rate, such a device can take advantage of a sleep mode. After measuring the temperature and transmitting information to the system, the sensor goes into low power mode where it consumes close to zero power. In this case, alkaline galvanic cells can be used, especially if the sensors need to be placed across a wide area. Motion and light sensors, on the other hand, must work constantly and respond quickly to external events. They require larger batteries. If they are not placed indoors, it is possible to feed them from solar panels, which will charge the lithium-ion battery during the day. In the dark, the sensor will be battery-powered.
Factors to be considered before making a choice
To choose a power source for a particular IoT system, one must first think about the cost, device dimensions, and convenience for the user. Usually, powering the device from the network is the cheapest for the manufacturer. However, this can often lead to additional costs spent on cables and adapters and connection is not always ergonomic. The cost of acid batteries is lower, but their weight and size do not allow using them in most IoT devices. Lithium batteries do not have such disadvantages and due to their thin structure can be manufactured in different shapes, but will cost more. In some cases, even alternative power sources may be more beneficial.
IoT devices are often required to be invisible and easily integrated into the environment, for example, a smart switch should have a wider range of functionality but fit in the same box as a regular one. One should pick a power source depending on the situation. For example, smart tags, recently attracting renewed attention from big tech companies, are powered by a plain silver-zinc galvanic cell that can be replaced. It is simply not advisable to build a rechargeable battery in such a device because this will need to be done 2-3 times during the tag's lifetime, while the product cost will increase significantly. An example of an interesting industrial design is the smartwatch with a thermoelectric element drawing energy from body heat. Using solar panels to charge the watch is probably not very advisable, as it limits the working area of the watch and the watch will simply not work if covered by clothing.
Future of power supplies
All devices (not just IoT) need power, so tens of billions invested in research of new power sources and in increasing the efficiency of the existing ones. New generation solid-state batteries development and testing of new combinations of existing materials are in progress. For example, the addition of graphene to the lithium-ion battery structure significantly improves its characteristics. And graphene polymer batteries should have 10 times bigger power density than all existing ones. The only problem today is the high cost of producing graphene itself. Energy converters are becoming smaller and more efficient due to the integration of components. But there is an approach that can turn all power technologies upside down in a smart home or in a smart workplace. Nikola Tesla dedicated his life to experiments that would allow wireless transmission of electricity to devices. He was way ahead of his time, so still, not all of his concepts are implemented. Now, finally, experimental wireless power lines have been created, which have proven themselves well. Maybe, we just have to wait for this technology to come to our home.
Conclusions
Strange as it may seem, power is the foundation of smart solutions. No matter how innovative the device is, no matter what it does, it will stop working as soon as its source of energy is depleted. Modern technology is tackling this problem by looking into more energy-efficient electronics and new energy sources. And there are often issues of not only economic feasibility but also convenience or ecology. The future does not appear to be hopeless, as new approaches and principles are being mastered that will aid in the construction of not only smart but also environmentally friendly houses or other systems.
